-
PDF
- Split View
-
Views
-
Cite
Cite
Francesco Raimondi, Fiorella Migliaro, Letizia Capasso, Gaetano Ausanio, Massimo Bisceglia, Paolo Giliberti, Francesco Messina, Gennaro Salvia, Roberto Paludetto, Intravenous Magnesium Sulphate vs. Inhaled Nitric Oxide for Moderate, Persistent Pulmonary Hypertension of the Newborn. A Multicentre, Retrospective Study, Journal of Tropical Pediatrics, Volume 54, Issue 3, June 2008, Pages 196–199, https://doi.org/10.1093/tropej/fmm101
Close - Share Icon Share
Abstract
We have compared intravenous magnesium sulphate vs. inhaled nitric oxide in the therapy of moderate persistent pulmonary hypertension of the neonate. A retrospective collection of clinical data from 58 neonates was carried out in six neonatal intensive care units of Southern Italy sharing the same operational protocols. In our setting, both drugs were effective in treating moderate persistent pulmonary hypertension of the neonate but nitric oxide (NO) treatment resulted in much faster amelioration of oxygenation index, taken as a marker of the underlying condition. No significant difference was recorded in immediate or long-term complications. We conclude that, wherever NO facilities are not readily available, magnesium sulphate is a safe and cheaper alternative for first-line treatment of moderate persistent pulmonary hypertension of the neonate.
Introduction
Persistent pulmonary hypertension of the newborn (PPHN) is a life-threatening disease occurring in 1.9/1000 live births either as a primary condition or secondary to asphyxia, infection, pulmonary hypoplasia, etc. [1]. The affected newborns, mostly at term, are hypoxemic because of right-to-left shunts through the ductus arteriosus and foramen ovale caused by the high vascular pulmonary pressure.
PPHN is still a therapeutic challenge; a specific pulmonary vasodilator has long been sought and inhaled nitric oxide (iNO) seems to best fit the task [2]. A meta-analysis of 12 randomized controlled studies showed NO to improve outcome in hypoxemic term and near-term infants by reducing the incidence of ECMO [3]. Unfortunately, NO delivery requires dedicated, expensive facilities that may not be available in every neonatal intensive care unit (NICU). Intravenous magnesium sulphate has been shown to treat effectively PPHN in non-randomized, small series of patients in developing countries [4,5]; although the latter represents a cheap and readily available alternative to NO, it is thought to be less effective and safe. The urge for comparative data has been stated in a recent Cochrane review [6]. However, given the great body of data on the efficacy and safety of NO and the different operational contexts, prospective trials of the two drugs in PPHN are hardly forecoming.
We have gathered the data of PPHN patients treated with either NO or magnesium sulphate of six centres in Southern Italy recognizing common drug delivery protocols.
Patients and Methods
A retrospective, multicentric analysis was performed between January 2003 and December 2004 in six-level III NICUs: University of Naples Federico II, Caserta City Hospital, Evangelic Foundation Betania Hospital of Naples, Monaldi Hospital of Naples, Fatebenefratelli Hospital of Naples, Crotone City Hospital. Fifty-eight term or near-term inborn infants with hypoxia due to PPHN were enrolled in the study. Babies with congenital malformations were excluded from the study.
The clinical diagnostic criteria of PPHN was a persistent hypoxemia (PaO2 < 50 mmHg at a fraction of inspired oxygen of 1.0) despite adequate ventilatory support. Colour flow Doppler echocardiography was performed in all enrolled cases to exclude congenital heart disease and to show evidence of right-to-left or bidirectional shunting at the ductus arteriosus or at the foramen ovale. In the same session, an estimated pulmonary artery pressure greater than systemic pressure by assessment of tricuspid regurgitation or ventricular septal position was documented. After the diagnosis of PPHN, treatment was initiated with either NO or magnesium sulphate depending on the individual NICU policy. Before and during therapy, infants were stabilized and vital signs were closely monitored. Ventilatory settings including fraction of inspired oxygen (FiO2), respiratory rate, peak inspiratory pressure (PIP), positive end expiratory pressure (PEEP) and mean airway pressure (Paw) were recorded at hourly intervals. Pre- and post-ductal saturation were continuously monitored. Adequate ventilatory support was given and surfactant was administered in the case of alveolar disease.
All babies were started on a dopamine and dobutamine infusion of 5 µg/kg/min and the dose was titrated depending upon the changes in blood pressure up to a maximum of 10 µg/kg/min. Mean arterial blood pressure was maintained between 40 and 45 mmHg. Arterial blood gas measurements were obtained from an indwelling catheter at hourly for the first 8 h after admission then every 6–12 h as per physician order. Serum calcium and blood glucose levels were recorded as frequently as required. No infant was put under systemic alkalinization.
Oxygen index (OI) was calculated using the following formula:
OI = [Mean airway pressure (Paw) × Fraction of inspired oxygen (FiO2) × 100]/oxygen partial pressure (PaO2).
A progressive OI decrease to 20 was used to define a responder to treatment.
Thirty out of 58 patients received iNO therapy. All the patients had an OI >30 despite maximal ventilatory setting, inotropic drugs and therapy for alveolar disease. The starting NO dose was of 5 ppm, increased by 5 ppm every 15 min until PaO2 increased of 30 mmHg; maximum iNO dose that the therapeutic schedules allowed to be reached was 40 ppm if previously unresponsive. After 4 h of stability, weaning from iNO therapy was accomplished using 2 ppm decrements every 2 h and then stopped. Nitrous oxide, nitric oxide (NO) concentration and metahaemoglobin levels were determined every day during the treatment.
Twenty-eight patients received MgSO4 infusion. A loading dose of 200 mg/kg of MgSO4 was given intravenously over 20 min, followed by a continuous infusion at the rate of 50 mg/kg/h as in the study by Chandran and coworkers [7]. Magnesium level was monitored twice daily. Once satisfactory PaO2 levels were achieved, ventilator settings were gradually reduced.
All infants were enrolled in the University of Naples ‘Federico II’ follow-up programme and up to the age of 24 months were periodically examined by a team composed by a staff neonatologist, a paediatric neurologist and a child development psychologist.
Data were analysed with a commercial computer software (SPSS, SPSS Inc., Chicago, IL). Given the small number of patients, only descriptive statistics were used.
Results
No differences between the groups were seen about gestational age, gender and primary diagnosis (Table 1). Average starting OI was between 30 and 40 in both groups.
Main characteristics of the treatment groups
| . | Magnesium sulphate (n = 28) . | Nitric oxide (n = 30) . |
|---|---|---|
| Gestational age (weeks) | 39.1 ± 1.1 | 38.7 ± 1.3 |
| Sex (M/F) | 12/16 | 15/15 |
| Primary diagnosis | ||
| ID | n = 8 | n = 9 |
| SAM | n = 14 | n = 15 |
| P | n = 6 | n = 6 |
| Mean age at the start of therapy (hours) | 10 h (range 2–36 h) | 14 h (range 6–48 h) |
| Duration of treatment | 30 h (range 12 h to 3 days) | 27 h (range 13 h to 9 days) |
| Responders | 27/28 (97%) | 30/30 (100%) |
| Side effects | Systemic hypotension n = 1 | None |
| . | Magnesium sulphate (n = 28) . | Nitric oxide (n = 30) . |
|---|---|---|
| Gestational age (weeks) | 39.1 ± 1.1 | 38.7 ± 1.3 |
| Sex (M/F) | 12/16 | 15/15 |
| Primary diagnosis | ||
| ID | n = 8 | n = 9 |
| SAM | n = 14 | n = 15 |
| P | n = 6 | n = 6 |
| Mean age at the start of therapy (hours) | 10 h (range 2–36 h) | 14 h (range 6–48 h) |
| Duration of treatment | 30 h (range 12 h to 3 days) | 27 h (range 13 h to 9 days) |
| Responders | 27/28 (97%) | 30/30 (100%) |
| Side effects | Systemic hypotension n = 1 | None |
ID, idiopatic; MAS, meconium aspiration syndrome; P, pneumonia.
Main characteristics of the treatment groups
| . | Magnesium sulphate (n = 28) . | Nitric oxide (n = 30) . |
|---|---|---|
| Gestational age (weeks) | 39.1 ± 1.1 | 38.7 ± 1.3 |
| Sex (M/F) | 12/16 | 15/15 |
| Primary diagnosis | ||
| ID | n = 8 | n = 9 |
| SAM | n = 14 | n = 15 |
| P | n = 6 | n = 6 |
| Mean age at the start of therapy (hours) | 10 h (range 2–36 h) | 14 h (range 6–48 h) |
| Duration of treatment | 30 h (range 12 h to 3 days) | 27 h (range 13 h to 9 days) |
| Responders | 27/28 (97%) | 30/30 (100%) |
| Side effects | Systemic hypotension n = 1 | None |
| . | Magnesium sulphate (n = 28) . | Nitric oxide (n = 30) . |
|---|---|---|
| Gestational age (weeks) | 39.1 ± 1.1 | 38.7 ± 1.3 |
| Sex (M/F) | 12/16 | 15/15 |
| Primary diagnosis | ||
| ID | n = 8 | n = 9 |
| SAM | n = 14 | n = 15 |
| P | n = 6 | n = 6 |
| Mean age at the start of therapy (hours) | 10 h (range 2–36 h) | 14 h (range 6–48 h) |
| Duration of treatment | 30 h (range 12 h to 3 days) | 27 h (range 13 h to 9 days) |
| Responders | 27/28 (97%) | 30/30 (100%) |
| Side effects | Systemic hypotension n = 1 | None |
ID, idiopatic; MAS, meconium aspiration syndrome; P, pneumonia.
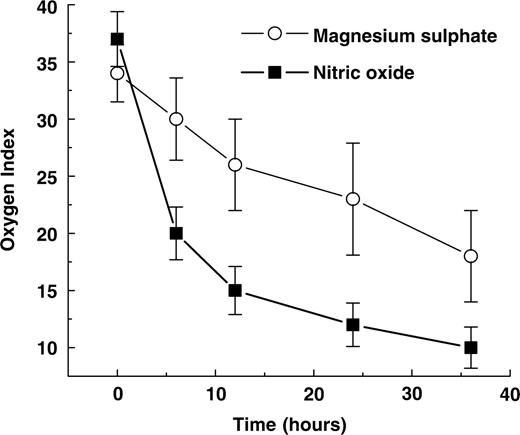
In our study, 30/30 patients (100%) responded to iNO therapy. A rapid improvement in oxygenation was achieved since average OI decreased to 20 after 6 h of treatment (Fig. 1) with a maximal iNO dose up to 20 ppm and kept a downward trend. No side effects were described during the treatment with NO. Metahaemoglobin levels were less than 5%, nitrous oxide and NO concentration were maintained at levels less than 2 ppm. Of 28 patients, 27 patients (97%) responded to MgSO4 treatment but the improvement in oxygenation was slower than in the NO group (Fig. 1). A decrement of the average OI to 20 was not detected before 36 h of treatment although no patient showed any trend to rebound. Systemic hypotension was reported in a single patient where MgSO4 was discontinued and treated with prostacyclin. We considered him as non-responder. Serum magnesium level in all cases was between 4 and 5.5 mmol/l. For both groups, no significant anomalies were detected by head ultrasound at discharge. Throughout the first 18 months, all infants showed a normal physical growth, neurological examination and achievement of the major psychomotor milestones.
Discussion
As a known vasodilator, MgSO4 was experimented in the nineties in a few small reports for the treatment of PPHN without significant side effects [4,5]; then, the relative success of NO reduced the attention on MgSO4 before those centres able to conduct a randomized trial had performed one. Despite a broad use of MgSO4 for PPHN, the soundest evidence of its efficacy is a single centre series on 12-term and near-term infants with severe PPHN treated with MgSO4 by Chandran et al. [7]; these authors recommended MgSO4 as first-line treatment wherever NO facilities were not easily available. To gain further insights on this potential lifesaving therapy, we have performed the first comparative, albeit retrospective, study on magnesium sulphate vs. iNO for PPHN treatment. We provide the largest series of PPHN cases treated with magnesium sulphate currently in the literature. Unlike previous reports from a single unit, our data come from the combined experience of six NICUs sharing the same operational protocols. In our setting, both drugs were equally effective in treating PPHN with a relevant number of responders and no substantial difference in either immediate or long-term side effects. However, NO was significantly faster in improving systemic oxygenation, while MgSO4 took much longer to act. The latter is a piece of information previously unreported in the literature and of clinical significance. Our data must be interpreted with caution as only infants with moderate (i.e. with a starting OI between 30 and 40) PPHN were enrolled. The reasons for this limitation are not entirely clear. More severe PPHN is often linked to surgical malformations such as congenital diaphragmatic hernia. In Southern Italy, such cases are strongly centralized in one NICU whose database was unavailable to us.
In conclusion, MgSO4 is a safe and cheaper alternative to iNO in the first-line treatment of moderate PPHN. Now that other inexpensive drugs have been recommended for this purpose (e.g. sildenafil) [8], we eagerly await further comparative trials.
Acknowledgement
The authors are grateful to the following colleagues for their contributions to the work: Luigi Falco, MD Ospedale Civile di Caserta, Caserta, Italy and Donato Zappulli, MD Ospedale Fatebenefratelli, Napoli, Italy



Comments